Pharmac is funding two new free medicines that replace some old Type-2 Diabetes treatments.
Call your doctor right now to see if you are eligible.
If you are eligible, these new medications will
reduce your risk of needing insulin[1], and
reduce your risk of dying from heart attack or kidney failure[2].
What you need to know
- In 2019, a long-term study[3] of diabetes care in South Auckland found that diabetes was not under control in many patients, and blood glucose levels had not improved for 10 years. This is one of the factors that leads to excess early death and progress to kidney failure in the community.
- Treatment of diabetes in New Zealand has not changed for 20 years. Current medicines do not prevent the important diabetes complications – early death and progress to kidney failure.
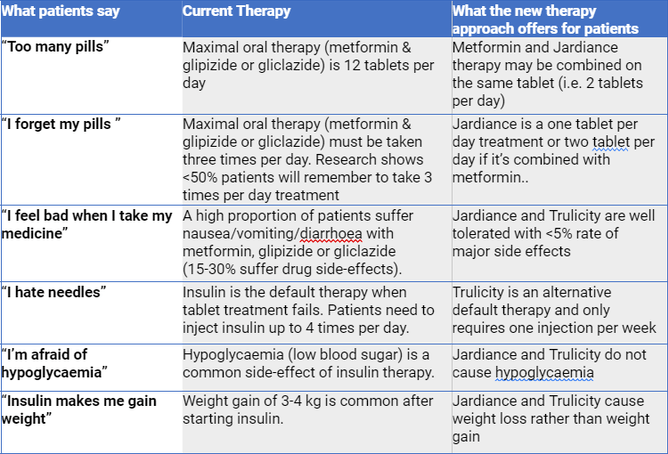
- Compared to the old ones, the new diabetes medicines are easy to take (less pills & injections per day), cause weight loss rather than weight gain, and cause less side effects.
- PHARMAC agreed late last year to pay for the two new types of treatment, Jardiance (SGLT2 inhibitor) and Trulicity (GLP1 receptor agonist). That means they’re free to patients.
- The new medicines are only available by “Special Authority”. That means a doctor has to apply on behalf of the patient.
- To be eligible for new medicines, the patient needs to meet the following criteria:
- Patient has type 2 diabetes; and
- Any of the following:
- Patient is Māori or any Pacific ethnicity; or
- Patient has pre-existing cardiovascular disease or risk equivalent*; or
- Patient has an absolute 5-year cardiovascular disease risk of 15% or greater according to a validated cardiovascular risk assessment calculator; or
- Patient has a high lifetime cardiovascular risk due to being diagnosed with type 2 diabetes during childhood or as a young adult; or
- Patient has diabetic kidney disease**; and
- Target HbA1c (of 53 mmol/mol or less) has not been achieved despite the regular use of at least one blood-glucose lowering agent (e.g., metformin, vildagliptin or insulin) for at least 3 months; and
- Treatment will not be used in combination with a funded [GLP-1 agonist/SGLT-2 inhibitor] (deleted as appropriate).
Patient perspectives
Here’s why the Diabetes Foundation Aotearoa argued for a completely new treatment regime.
- JARDIANCE (empagliflozin 10mg and 25mg tablets) is a Prescription Medicine for the treatment of adult patients with Type 2 diabetes, funded under special authority criteria – restrictions apply. Normal doctor fees and prescription charge will apply. Product information and Consumer Medicine Information (CMI) can be obtained from www.medsafe.govt.nz . All medicines including Jardiance® has risks and benefits. Ask your doctor if Jardiance® is right for you. If symptoms continue or you have side effects see your doctor. Jardiance® is a registered trademark of Boehringer Ingelheim.
- TRULICITY® (dulaglutide 1.5 mg/0.5 mL solution for injection, pre-filled pen [autoinjector]) is a Prescription Medicine for the treatment of adult patients with Type 2 diabetes, funded under special authority criteria – restrictions apply. Normal doctor fees and prescription charge will apply. Product information and Consumer Medicine Information (CMI) can be obtained from www.medsafe.govt.nz . All medicines including Trulicity® has risks and benefits. Ask your doctor if Trulicity is right for you. If symptoms continue or you have side effects see your doctor. Trulicity® is a registered trademark of Eli Lilly and Company (NZ) Limited.
The DCSS audit
- The Diabetes Foundation Aotearoa administered an audit of more than 46,000 patients with Type 2 diabetes in South Auckland between 1994 and 2018. The purpose of the audit was to assist GPs to improve standards of care.
- The audit was managed by the Diabetes Care Support Services (DCSS), a group of diabetes health professionals, and funded by the Counties Manukau District Health Board.
- When the audit finished in 2018, it was evaluated by Professor David Simmons, Distinguished Professor of Medicine West Sydney University.
- Professor Simmons reported that less than 50% of patients achieved the target glucose control (HbA1c <53 mmol/mol). Māori and Pacific patients had the poorest glucose control - worse than any other population in the Western World (40% of Māori and 30% of Pacific patients achieved the target)
- Microalbuminuria, a precursor of early kidney damage and dialysis, remained at high levels throughout the 25 years, affecting 20% of NZ Europeans and 35% of Māori and Pacific patients.
- Compared to non-diabetic individuals, mortality rates of diabetes patients remained high compared with the general population at 2-fold among NZ Europeans, 4-fold among Māori and over 3-fold among Pacific patients.
- Hospitalisation rates primarily due to kidney diseases increased, with rates of 7-9% among European and Pacific patients and over 11% among Māori patients.
- Professor Simmons concluded blood glucose was severely under-managed and likely to have caused the high levels of premature death and diabetic kidney diseases. This is likely due to no access to modern glucose lowering medications SGLT-2 inhibitors (Jardiance)and GLP-1 receptor agonists (Trulicity), the only drugs shown to reduce death.
Findings of the DCSS audit were presented at public meetings in 2019 and 2020 and published in the Lancet medical journal in 2019 and the New Zealand Medical Journal in 2020.
TextWebsiteVer5-8 July 2021
[1] Decreased likelihood of receiving insulin when taking Empagliflozin is from EMPA-REG trial.
[2] Relative risk reduction (RRR) for death from diabetes for SGLT-2 inhibitors is from EMPA-REG trial. RRR for death from diabetes for GLP-1 receptor agonist is from LEADER trial.
[3] The DCSS Audit was funded by a grant from the Middlemore Foundation Trust, and conducted by Dr David Simmons, Distinguished Professor of Medicine at West Sydney University and an international expert in diabetes and integrated care.